Thousands Could Die From Blood Clot Device, Potential FDA Fraud
October 30, 2015
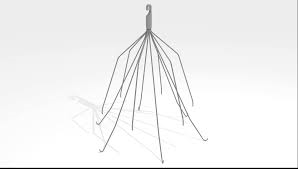
Inferior vena cafa filter lawyers (IVC filter lawyers) at Morrow & Sheppard assist victims nationwide who have suffered serious injuries caused by IVC filters, devices intended to protect against blood clots, but that have a known tendency to fail and “migrate” into the heart, causing debilitating injury or even death.
What Is The Inferior Vena Cava?
The inferior vena cava is one of two large veins that carries deoxygenated blood from the body into the heart’s right atrium.
What Is An IVC Filter?
An inferior vena cava filter or “IVC filter” is a medical device implanted into the inferior vena cava. The purpose of the device is to prevent blood clots, also known as pulmonary emboli or “PE”.
The FDA has described IVC filters as follows:
“IVC filters are small, cage-like devices that are inserted into the inferior vena cava (the main vessel returning blood from the lower half of the body to the heart) to capture blood clots and prevent them from reaching the lungs. IVC filters are frequently placed in patients at risk for pulmonary embolism (a blood clot in the lungs) when anticoagulant therapy cannot be used or is ineffective. Some patients may require long-term protection from PE, and implantation of permanent IVC filters is often performed in these cases. Others only require short-term protection, in which case retrievable IVC filters are typically used, as these devices have the option to be removed once the patient’s risk of PE subsides.”’
IVC Filters Do Not Work
IVC filters have never been shown to be effective.
The only study that has shown any benefit from IVC filters indicated that even if they reduce blood clots, the devices increase the risk of potentially dangerous deep vein thrombosis or “DVT.”
IVC Filter Deadly Danger: They Can “Migrate To” And Puncture The Heart
IVC filters have a tendency to dislodge, break, or fracture, then “migrate to” and pierce or puncture the heart.
Broken filters can also cause embolization (entry into bloodstream and blockage).
Real-life examples of IVC failures include:
- An Illinois man whose Bard Recovery IVC filter broke and migrated to his heart, causing permanent complications.
- A woman from Illinois whose Bard G2 IVC filter splintered pieces into her heart. Pieces are still in her lungs.
IVC Filters Cause Death
Some IVC filter victims have paid the ultimate price.
The failure of C.R. Bard’s “Recovery” IVC filter alone has caused 27 reported deaths. Of course that figure does not include other models, or IVC filters produced by other manufacturers.
Most frightening, there are thousands of these ticking time bombs currently residing in unsuspecting victims. The more time that passes, the greater the likelihood of a potentially deadly failure.
Who Manufacturers IVC Filters?
11 companies manufacture IVC Filters in the United States, including:
- ALN Implants Chirurgicaux (ALN Vena Cava Filters)
- Argon Medical Devices (Option Elite Retrievable Vena Cava Filter designed and manufactured by Rex Medical)
- B Braun Interventional Systems (VenaTech LP Vena Cava Filter)
- Bard Peripheral Vascular (DENALI Vena Cava Filter System)
- Cook Incorporated (Cook Günther Tulip Vena Cava Filter)
- Cordis Corporation (Cordis OptEase Retrievable Vena Cava Filter/ Cordis TrapEase Vena Cava Filter)
- Volcano Corporation (Crux Vena Cava Filter System)
IVC Filter Cover Up? Former C.R. Bard Specialist Claims Forgery.
In September 2015, NBC News revealed that C.R. Bard may have actively concealed the dangers of IVC filters. Worse, a former employee of C.R. Bard revealed that the company forged her signature to obtain government approval of the device.
The NBC News report states that C.R. Bard submitted IVC filters for Food and Drug Administration (“FDA”) approval in 2002. The FDA rejected the application.
C.R. Bard then hired an FDA regulatory specialist, Kay Fuller, to help push the device through the approval process. Fuller immediately raised concerns about the device, including concerns about why the company would not show her performance testing results, and whether it was reasonable to claim the devices were safe based on a very small and iffy human clinical trial.
As Fuller told NBC, she was “shocked” when C.R. Bard responded to her concerns by stating she would be kicked off the team if she continued to make waves. She resigned and reported the issues to the FDA. According to Fuller, C.R. Bard then forged her signature on a new FDA application to obtain approval for the device.
Initial 2010 FDA Warning
In August 2010, the Food and Drug Administration (“FDA”) issued the first warning regarding IVC filters. The warning was issued to “implanting physicians and clinicians responsible for the ongoing care of patients with inferior vena cava (IVC) filters. Includes interventional radiologists, interventional cardiologists, vascular surgeons, emergency room physicians (trauma), bariatric surgeons, orthopedic surgeons, primary care physicians.”
The warning states:
“IVC filter usage has increased rapidly during the past thirty years. In 1979, 2,000 IVC filters were used, while in 2007, almost 167,000 filters were implanted, and the market for IVC filters is only expected to increase, with an estimated 259,000 IVC filters to be deployed in 2012 (Smouse and Johar, Endovascular Today, February 2010).
Since 2005, the FDA has received 921 device adverse event reports involving IVC filters, of which 328 involved device migration, 146 involved embolizations (detachment of device components), 70 involved perforation of the IVC, and 56 involved filter fracture. Some of these events led to adverse clinical outcomes in patients. These types of events may be related to a retrievable filter remaining in the body for long periods of time, beyond the time when the risk of PE has subsided.
The FDA is concerned that these retrievable IVC filters, intended for short-term placement, are not always removed once a patient’s risk for PE subsides.
Known long term risks associated with IVC filters include but are not limited to lower limb deep vein thrombosis (DVT), filter fracture, filter migration, filter embolization and IVC perforation.
FDA recommends that implanting physicians and clinicians responsible for the ongoing care of patients with retrievable IVC filters consider removing the filter as soon as protection from PE is no longer needed.
FDA encourages all physicians involved in the treatment and follow-up of IVC filter recipients to consider the risks and benefits of filter removal for each patient. If a patient has a retrievable IVC filter that should be removed based on his or her individual risk/benefit profile, the primary care physician and/or those providing ongoing patient care should refer the patient for IVC filter removal when feasible and clinically indicated.
As part of developing our final position, FDA reviewed the literature and is conducting quantitative decision analysis modeling to evaluate the change in the risk/benefit profile after retrievable IVC filter implantation over time. More information about FDA’s decision analysis model including risk/benefit implantation timeframe suggestions will be made available in an update to this communication as well as in a future publication in a peer-reviewed medical journal.”
2014 Updated IVC Filter Warning
In 2014, the FDA updated its IVC filter warning:
“The FDA developed a quantitative decision analysis using publicly available data available in the medical literature to assess whether there is a time period during which the risk of having an IVC filter in place is expected to outweigh the benefits. The decision analysis (Decision Analysis of Retrievable Inferior Vena Cava Filters in Patients without Pulmonary Embolism) was published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders in October 2013. The mathematical model suggested that if the patient’s transient risk for pulmonary embolism has passed, the risk/benefit profile begins to favor removal of the IVC filter between 29 and 54 days after implantation.
Although the results of the decision analysis provide important insight for retrievable IVC filters, the FDA is requiring collection of additional clinical data for currently marketed IVC filters in the United States. The studies will address safety questions that remain unanswered for both permanent and retrievable filters. Manufacturers were given two options for obtaining the data. Some manufacturers are participating in the PRESERVE (PREdicting the Safety and Effectiveness of Inferior Vena Cava Filters) study, an independent national clinical study that will examine the use of IVC filters in the prevention of pulmonary embolism. Other manufacturers are conducting postmarket surveillance (522 Studies). The data gathered from the PRESERVE study and the 522 studies will help the FDA, manufacturers and health care professionals assess the use and safety profile of these devices, understand evolving patterns of clinical use of IVC filters and ultimately improve patients care.”
IVC Injury Filter Lawyers Assist Victims Nationwide
Morrow & Sheppard assist victims and family members affected by IVC filter injuries and wrongful deaths nationwide.
Please call us now at 800-489-2216 for a free, confidential consultation to discuss your legal rights.
- Home
- |
- Dangerous Drugs & Products
- |
- Thousands Could Die From Blood...















































