How Morcellators Spread Cancer
June 14, 2015

Thousands of women who had hysterectomies and myomectomies have needlessly contracted uterine cancer or benign tumors.
The reason? A dangerous medical device called a “power morcellator” was used in these procedures. As is discussed below, morcellators spread or “seed” cancer and tumors throughout the body. Manufacturers were notified of their dangers years ago.
Outline
This article will discuss the following topics:
- What Is A Power Morcellator?
- The Problem With Morcellators: They Spread Cancer
- Who Is Ethicon?
- April 2014: Initial FDA Warning Regarding Morcellators
- Early July 2014: Official Panel Discusses Risks of Morcellators
- Late July 2014: Ethicon Withdraws Morcellators
- May 2015: FBI Opens Investigation Into Ethicon Morcellators
- Our Houston Injury Lawyers Represent Morcellator Victims Nationwide
- Other Morcellator Articles
What Is A Power Morcellator?
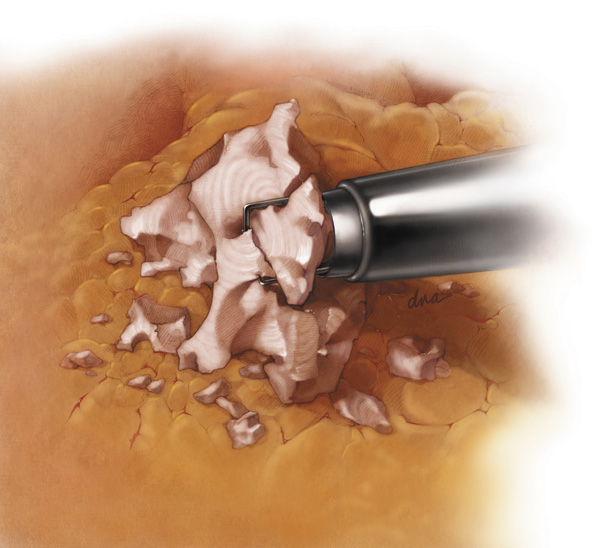
A morcellator is used to chop up and remove tissue during surgery.
In a laparoscopic hysterectomy, the device minces up (“morcellates”) the uterus into small pieces that are easier to remove.
Laparoscopic power morcellators were sold as revolutionary devices that would enable doctors to perform hysterectomies and myomectomies in a much less invasive manner.
Specifically, rather than the 3- to 7-inch incisions typically required to remove an intact uterus during a hysterectomy, incisions from power morcellators are less than 4 centimeters (1.5 inches) in size. Use of morcellators was also thought to shorten patient recovery periods.
Laparoscopic power morcellators could also be used, so said manufacturers, to safely remove benign uterine growths called fibroids leftover from previous hysterectomies, a procedure called a myomectomy.
The Problem With Power Morcellators: They Spread Cancer
The problem with power morcellators is that they significantly increase a patent’s risk of contracting Leiomyosarcoma (LMS), malignant cancer involving tumors that develop in smooth muscle tissue.
LMS is a particularly dangerous form of cancer because it can remain dormant for extended periods of time, and often recurs after several years. Worse, it is generally not responsive to chemotherapy or radiation.
So how do power morcellators cause cancer? In the course of mincing uterine tissue, morcellators spread and leave behind small bits of tissue. If these tissue fragments contain any cancer-causing cells, they can spread or “seed” rapid cancer growth.
Who Is Ethicon?
![]()
Ethicon Inc. is a subsidiary of Johnson & Johnson. The company designs products for use in surgeries.
Ethicon’s products are intended to assist with a variety of surgery-related functions, including bodily access, ligation, hernia repair, surgical stapling, uterine and pelvic surgery, and wound closure.
The company claims “[o]ur story is about shaping the future of surgery so every patient, every family, every community receives the care they need for healthier lives.”
Several Ethicon products have caused injury in the past, including pelvic mesh.
Johnson & Johnson sold over $74 billion worth of products in 2014. That same year, the company made $7.9 billion in profit on medical devices alone.
CEO Alex Gorsky recently boasted that the company has consistently “delivered solid financial results,” notwithstanding the millions of dollars in settlements it has paid to victims in lawsuits arising from the dangerous products the company has manufactured.
Ethicon manufactured most of the power morcellators used to perform hysterectomies and myomectomies.
April 2014: Initial FDA Warning Regarding Morcellators

On April 27, 2014, the Food and Drug Administration (FDA) issued an official safety communication regarding laparoscopic uterine power morcellation in hysterectomies and myomectomies.
The FDA’s warning was directed to:
- Health Care Providers
- Medical Professional Associations
- Cancer Advocacy Organizations
- Health Care Facilities/Hospitals
- Women with Symptomatic Uterine Fibroids who are Considering Surgical Option
- Manufacturers of Devices used for Minimally Invasive Surgeries
The FDA warned:
If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient’s likelihood of long-term survival. For this reason, and because there is no reliable method for predicting whether a woman with fibroids may have a uterine sarcoma, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.
The FDA also resolved to undertake the following emergency actions to investigate and ensure the safety of morcellators: (1) instruct manufacturers to immediately review their warning labels; (2) convene a meeting of the Obstetrics and Gynecological Medical Device Advisory Committee to discuss issues with the devices; and (3) thoroughly “review adverse event reports, peer-reviewed scientific literature, and information from patients, health care providers, gynecologic and surgical professional societies, and medical device manufacturers.”
Early July 2014: Official Panel Discusses Risks of Morcellators
At the FDA’s request, the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee held a two-day public meeting on July 10-11, 2014 to discuss the safety of morcellators.
Specifically, the Committee was asked to evaluate “the safety of laparoscopic power morcellator devices as it pertains to their potential to disseminate and upstage a confined, but undetected (occult) uterine malignancy during laparoscopic hysterectomy or myomectomy.”
After two days, the panel concluded that even based on the limited and still-developing information available at the time, “the impact on the individual patient is very large.”
Late July 2014: Ethicon Withdraws Morcellators
On July 30, 2014, Ethicon issued an “Urgent Message” officially withdrawing morcellators from the market.
Ethicon admitted that it “believes that a market withdrawal of Ethicon Morcellation Devices is the appropriate course of action.”
May 2015: FBI Opens Investigation Into Ethicon Morcellators
On May 27, 2015, news broke that the Federal Bureau of Investigation (FBI) is investigating whether or not Ethicon knew more than it told everyone about the dangers of morcellators.
As of this writing, the investigation is still ongoing.
Our Morcellator Injury Lawyers Represent Victims Nationwide
If you or a loved one has been diagnosed with uterine cancer following hysterectomy or myomectomy involving a morcellator, please contact our morcellator injury lawyers for a free, confidential consultation to discuss your legal rights.
The Texas morcellator injury lawyers at Morrow & Sheppard are based in Houston, but we handle products liability lawsuits for victims around the country.
Other Morcellator Articles
The Man Who Tried To Blow The Whistle: Texas Morcellator Lawyers
Congress Debates Power Morcellator Injuries
- Home
- |
- Dangerous Drugs & Products
- |
- How Morcellators Spread Cancer















































